Abstract
The healing of wounds is a complex, multifactorial process that remains incompletely understood. Health providers in Europe face an increasing number of costly chronic wounds which profoundly affect patients' quality of life. Wound healing is highly a dynamic and heterogeneous process, which impedes the dissection of the contribution of the different cell lineages by bulk transcriptomics analysis.
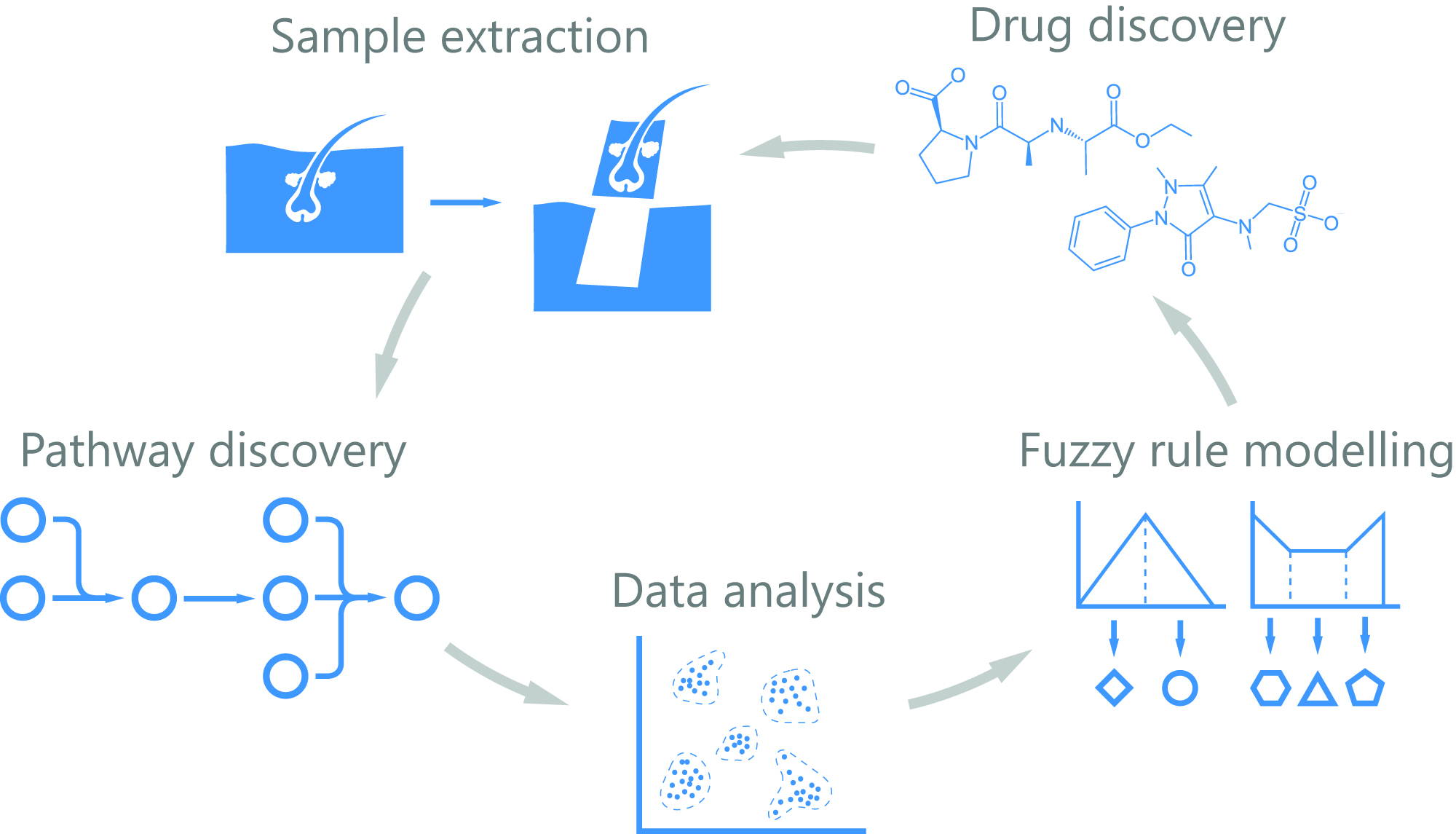
In addition, a static snapshot by single cell analyses might be uninformative. 4D-HEALING will map the four spatiotemporal dimensions of human wound healing in an unprecedented way: the transcriptome of thousands of cells from an in vivo monitored human wound will be analysed by RNAseq at the single cell level. We will model the transcriptional dynamics of cell lineages involved in all phases of healing, from injury to resolution. To gather positional information we propose a novel computational tool to predict "cell docking", based on the probability of cell-to-cell interactions and produce a neighbouring map that will be validated by annotating positional information of cells (multiplexed hybridization approach).
The emerging knowledge will provide a 4D map of the cell types involved in human wound healing allowing us to create a dynamic mathematical model, to disentangle the crosstalk among cell signalling and interactions, transitional states and position during the healing process. These models integrated together with FDA-approved drug databases will permit informed choices on molecular candidates that can be pharmacologically modulated. Candidate compounds will be tested in ex vivo human skin organ cultures mimicking acute and chronic wound environments. The resulting leads will undergo standard drug development programs.
The project will deliver data-driven, comprehensive understanding of the human wound healing process and in vitro proof of concept of a novel topical therapy to enhance chronic wound healing. Understanding wound healing will shed light to regenerative medicine.